Introduction: Lymphodepletion (LD) is crucial for the expansion and persistence of CAR T cells. Fludarabine in combination with cyclophosphamide (FC) is the LD standard regimen. A national fludarabine shortage in 2022 necessitated the exploration of alternative LD regimens. Bendamustine (benda) is a purine analog and an alkylating chemotherapy that has been used as an LD agent for tisagenlecleucel (Ghilardi et al.,2022). However, there is no data for benda LD in other commonly used CART products for relapsed or refractory non-hodgkin lymphoma (rel/ref NHL). Early safety, efficacy, and expansion kinetics data were presented previously (Bharadwaj, ASH 2022). We are now reporting long-term efficacy and immune reconstitution data.
Methods: 84 consecutive patients with relapsed/refractory LBCL treated with axi-cel were included in this single-institution study. 27 patients who received Bendamustine lymphodepletion (90mg/m2 on days -4 and -3) were compared to a preceding 57-patient FC cohort. Response, survival, and cytopenia data were collected closest to the 12-month time point for all patients who had not progressed. Response was assessed using the Lugano 2014 criteria, while toxicities were evaluated using the CTCAE version 5.0 and the ASTCT grading. To reduce the influence of confounding variables in this study, a covariate balance propensity score weighting approach (IPTW) was implemented. Covariates included age, sex, presence of bridging therapy, IPI score at apheresis, number of prior therapy lines, absolute lymphocyte count at leukapheresis, prior auto SCT, and pre-lymphodepletion LDH, ALC, ANC, and platelet count. Continuous outcomes were compared using the Mann-Whitney U test, categorical outcomes using chi-square tests, and time-to-event outcomes using a log-rank test weighted in the IPTW sample. Statistical significance was considered when p < 0.05. All analyses were conducted in R 4.3.0.
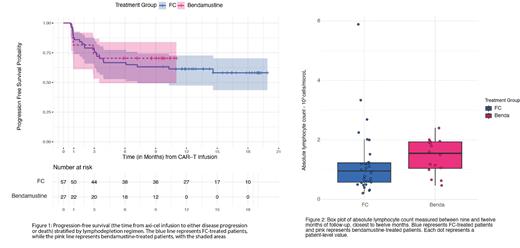
Results: There was no significant difference in the best complete response (CR) rate and best overall response rate (ORR) between the two groups, with a CR rate of 71.9% for FC and 74% for bendamustine (p = 0.182), and an ORR of 89.4% for FC and 85.1% for bendamustine (p = 0.502). For the FC group, 9-month progression-free survival (PFS) and overall survival (OS) were 63.16% (95% CI: 49.29% to 74.19%) and 78.95% (95% CI: 65.92% to 87.45%), respectively with a median follow-up of 15.5 months (IQR: 13.6, 16.8). 9-month PFS and OS point-estimates for the bendamustine group were 70.37% (95% CI: 49.4% to 83.94%) and 77.41% (95% CI: 56.41% to 89.18%), respectively, with a median follow-up of 9.5 months (IQR: 8.6, 9.8). No significant difference was found between groups in either 9 months PFS ( P=0.055) or 9 months OS ( P=0.6290). Median PFS and OS have not been achieved for either group [Figure 1]. Bendamustine was effective in achieving severe lymphodepletion, with a median absolute lymphocyte count (ALC) nadir of 0.11 K/ul (IQR: 0.04, 0.17) occurring within one day after axi-cel infusion. In comparison, the FC group had a lower median ALC nadir of 0.03 K/ul (IQR: 0.01, 0.06) occurring on the day of axi-cel infusion ( P=0.01). Both cohorts showed a similar pattern of lymphocyte recovery over 1, 3, and 6 months. Median ALC counts at 9-12 months post-infusion indicated better lymphocyte recovery in the bendamustine group with a median ALC of 1.55 K/ul (IQR: 1.04, 1.94) compared to 0.96 K/ul (IQR: 0.58, 1.23) in the FC group ( P=0.043) [Figure 2]. There was no significant difference in the recovery of absolute neutrophil count, hemoglobin, and platelets. Updated efficacy analysis and immune reconstitution data including T-cell, B-cell, and NK cell recovery will be reported at the time meeting, as all patients will have completed at least one year of follow-up by September 2023.
Discussion: Bendamustine lymphodepletion prior to axi-cel therapy showed comparable efficacy and safety outcomes to the standard FC regimen in relapsed/refractory LBCL patients. Bendamustine demonstrated effective lymphodepletion and better lymphocyte recovery at 9-12 months post-infusion compared to FC cohort. These findings suggest that bendamustine may be an acceptable alternative lymphodepleting regimen. Prospective studies, ideally randomized control trial, are warranted.
Disclosures
Hamilton:Kite Pharma: Other: Advisory Board. Smith:A28: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy. Frank:BRVLH: Consultancy; Cargo Therapeutics: Consultancy, Other: Travel Support; EcoR1: Consultancy; Adaptive Biotechnology: Consultancy; Roche/Genentech: Current holder of stock options in a privately-held company; Kite, a Gilead Company: Research Funding; Allogene: Consultancy; Gilead Sciences: Consultancy, Other: Travel Support. Miklos:2Seventy Bio: Research Funding; Allogene: Research Funding; Adicet: Research Funding; Incyte: Consultancy, Honoraria; Miltenyi: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy; Adaptive Biotechnologies: Consultancy; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: rights to royalties from Fred Hutch for patents licensed to Juno, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Legend Biotech: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Research Funding; Navan Technologies: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Amgen: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; NA: Patents & Royalties: cGVHD patent holder for Ibrutinib as cGVHD therapy but no compensation; Novartis: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel support; Umoja: Consultancy, Honoraria; Bioline Rx: Membership on an entity's Board of Directors or advisory committees. Dahiya:Adaptive Biotechnologies: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Incyte: Consultancy; Bristol Myers Squibb: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal